Endocytosis-mediated mitochondrial transplantation: Transferring normal human astrocytic mitochondria into glioma cells rescues aerobic respiration and enhances radiosensitivity
Recently researchers in the Radiation Medicine Group at the Institute of Modern Physics, Chinese Academy of Sciences obtained new findings on starvation-induced endocytosis via NAD+-CD38-cADPR-Ca2+ signaling could be a new mechanism of mitochondrial transplantation to rescue aerobic respiration and attenuate the Warburg effect. This mechanism could be a promising approach for radiosensitization.
Emerging evidence indicates that reprogramming of energy metabolism involving disturbances in energy production from a defect in cellular respiration with a shift to glycolysis is a core hallmark of cancer. Alterations in cancer cell energy metabolism are linked to abnormalities in mitochondrial function. Mitochondrial dysfunction of cancer cells includes increased glycolysis, decreased apoptosis, and resistance to radiotherapy.
The origin of organelles is a debated step in eukaryotic evolution but contributed to the emergence of cellular complexity that characterizes modern eukaryotes. Although the endosymbiotic theory of the origin of mitochondria was suggested by Lynn Margulis in 1967, doubts continue about the theory because of the lack of direct evidence.
Researchers in Radiation Medicine Group found that mitochondria from HA could be transferred into starved U87 cells by simple co-incubation. Starvation treatment slowed the rate of glycolysis and decreased the transformation of NAD+ to NADH in U87 cells. A large amount of accumulated NAD+ was released into the extracellular space. CD38 is a member of the NAD+ glycohydrolase family that catalyzes the cyclization of extracellular NAD+ to intracellular cADPR. cADPR triggered release of Ca2+ to promote cytoskeleton remodeling and plasma membrane invagination. Thus, endocytosis involving isolated mitochondria internalization was mediated by NAD+-CD38-cADPR-Ca2+ signaling. Mitochondrial transfer enhanced gene and protein expression related to the tricarboxylic acid (TCA) cycle, increased aerobic respiration, attenuated glycolysis, reactivated the mitochondrial apoptotic pathway, inhibited malignant proliferation of U87 cells. Isolated mitochondria injected into U87 xenograft tumors also entered cells, and inhibited glioma growth in nude mice. Mitochondrial transplantation could enhance the radiosensitivity of gliomas in vitro and in vivo.
This study is the first to introduce mitochondrial transplantation into the field of tumor radiotherapy, and propose that autologous mitochondrial transplantation could be a therapeutic intervention that uses mitochondria from patients who are both the donor and recipient. Since mitochondria are processed outside the body and reintroduced into the same patient, this therapy would be of high specificity and would eliminate post-transplantation immune rejection. In the near future, every cancer patient will contribute their own mitochondrial biological agents.
This study was jointly supported by the National Natural Science Foundation of China, the Ministry of Science and Technology National Key R & D Project and the CAS “Light of West China” Program.
The research achievements have been published in Theranostics, 2019, 9(12): 3595-3607.
The article link is as follows: http://www.thno.org/v09p3595.htm
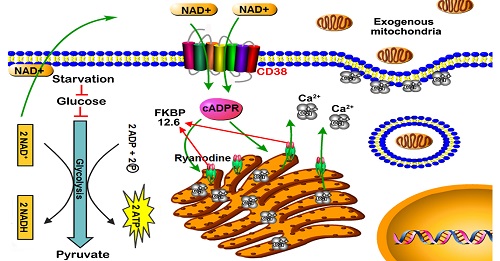
Fig 1. Schematic diagram of endocytosis-mediated mitochondrial transplantation via NAD+-CD38-cADPR-Ca2+ signaling
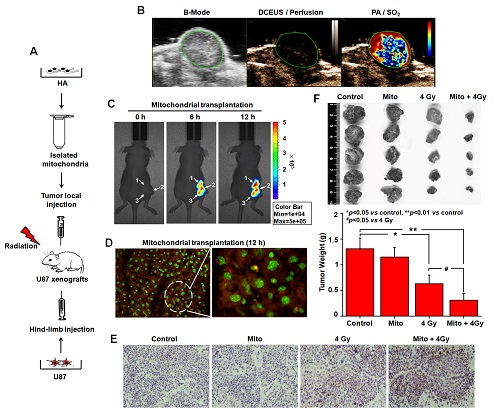
Fig 2. Effects of mitochondrial transplantation on the radiosensitivity of gliomas
Fig 3. The research achievements have been published in Theranostics



 甘公网安备 62010202000713号
甘公网安备 62010202000713号


