Important progress of mononuclear rhenium carbonyl complex in the gas phase
Recently, the systematic study on the Group-VII Re carbonyls formed in the gas-phase chemical reactions was carried out. This work was led by the researchers from nuclear chemistry group of IMP in collaboration with the researchers from RIKEN (Japan) and PSI (Switzerland). This work provided a good foundation for future investigation on the superheavy Bh carbonyl complexes.
Using short-lived radioisotopes produced at a heavy-ion accelerator RILAC in RIKEN, the short-lived Re isotopes were produced in the nuclear reactions of natGd(23Na,xn)172-177Re, and then pre-separated with a gas-filled recoil ion separator (GARIS). The evaporation residues recoiled from the target were ion-optically guided to the focal plane of the GARIS, and their carbonyls were synthesized in a mixture of inert gas and carbon monoxide. Volatile carbonyl complexes were formed in situ after losing the residual kinetic energy and then only neutral species can be transported through a Teflon capillary to a low temperature isothermal chromatography (IC) device for the measurement of the adsorption enthalpy. We also employed an improved laser-ablation time-of-flight mass-spectrometric (LA-ToF-MS) technique at IMP to study the carbonyl ions in the gas-phase. Metal carbonyl cations were produced in a molecular beam by laser vaporization in a supersonic expansion of helium seeded by CO gas. After passing through an extension pipe with increased reaction time and strengthened interaction, the final cation products were mass-analyzed in a modified time-of-flight spectrometer. With such a setup, we successfully identified the most stable products: the 18-e carbonyl ion [Re(CO)6]+, as well as the 17-e carbonyl ions [Os(CO)5]+ and [W(CO)5]-, which were formed in gas phases composed similar to our gas chromatography experiment. Their isoelectronic species – the 17-e neutral Re(CO)-5 – was proved to be stable in the inert gas atmosphere.
The above results were further confirmed with the theoretical methods. According to the molecular orbital theory and the ligand field theory, the mononuclear carbonyls of Group-VII are predicated to be 17-electron open-shell. Influenced by the relativistic effect and the Jahn-Teller effect, the Group-VII mononuclear carbonyls are reactive with low chemical yield, causing considerable difficulties of identification and characterization with traditional chemistry methods. Using density functional theory (DFT), our calculations on the relative energies and the FBDEs revealed that the coordination of CO with single-atom Re or Re+ are thermodynamically spontaneous in the gas phase. The final product were derived to be the 18-e [Re(CO)6]+ and the 17-e Re(CO)5.
The experimental observations with short-lived isotopes together with the theoretical analysis confirmed the kinetically fast formation of single-atom quantities of open-shell mononuclear Re(CO)5 in the CO-containing inert gas mixture. The adsorption enthalpy was deduced to be -42 kJ/mol on Teflon surface. The highest chemical yield was determined to be around 25% in the He/CO gas mixture. The above conclusions provided important information for the gas-phase chemical properties of Group-VII mononuclear carbonyl complexes, and laid the foundation for the next experimental and theoretical studies on Bh carbonyls in the future.
This work was financially supported by the National Natural Science Foundation of China. Related contributions have been published on the international journal Physical Chemistry Chemical Physics.
The web link of the published article: https://pubs.rsc.org/en/content/articlelanding/2019/cp/c8cp07844k/unauth#!divAbstract.
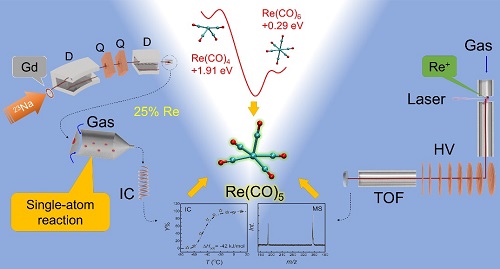
Figure . Schematic for the gas-phase chemistry study on mononuclear Re carbonyls (RILAC-GARIS+OLGA), LA-TOF-MS apparatus and the theoretical results



 甘公网安备 62010202000713号
甘公网安备 62010202000713号


