New Study Uncovers Key Mechanism Behind Superior Biological Effects of Heavy-ion Cancer Therapy
Heavy-ion therapy, one of the most advanced radiotherapy techniques, has proven to be more effective than conventional X-rays and proton radiation. However, the mechanisms behind this superior biological effectiveness remain unclear.
Published in Physical Review X on March 11, a new study has uncovered a key mechanism involving Intermolecular Coulombic Decay (ICD) in aqueous environments initiated by heavy-ion irradiation, providing insights into the aforementioned question.
The study was conducted by researchers from the Institute of Modern Physics (IMP), Chinese Academy of Sciences (CAS), in collaboration with researchers from Irkutsk State University, Heidelberg University, the University of Science and Technology of China, Xi'an Jiaotong University, and Lanzhou University.
In this study, the researchers investigated how heavy-ion radiation affects biomolecules at the molecular level. They developed an advanced supersonic gas jet technology to produce clusters of biomolecules attached to water molecules. Pyrimidine molecules, the fundamental structural units of DNA bases, were chosen to simulate DNA in the tissue environment and to study the decay mechanism after heavy-ion irradiation.
The experiments were performed at the experimental Cooler Storage Ring of the Heavy Ion Research Facility in Lanzhou and the 320 kV platform for multi-discipline research with highly charged ions. The researchers used carbon and iron ion beams to bombard the clusters of pyrimidine surrounded by water molecules.
For the first time, the study revealed that in living tissues irradiated by heavy-ion beam, inner-valence ionized water can transfer excitation energy to DNA molecules through the ICD process.
"This process not only leads to the ionization of the pyrimidine molecule with emission of a low-energy electron, but also initiates proton transfer between water molecules, resulting in the production of harmful secondary particles of hydroxyl radical and hydrated protons," said Prof. XU Shenyue from IMP, one of the corresponding authors of the study.
This finding challenges the long-held assumption that inner-valence ionized water cations decay through self-fragmentation, with their fragments indirectly affecting DNA. Instead, the observed mechanism demonstrated that these inner-valence ionized water cations can directly interact with DNA through ICD on the one hand and release harmful secondary particles near DNA on the other. This can greatly increase the risk of DNA double-strand breaks.
Furthermore, compared to other types of radiation, heavy-ion irradiation significantly increases the proportion of inner-valence ionization in water molecules, thereby dramatically enhancing the biological effectiveness.
"The observed mechanism is an important factor contributing to the superior biological effects of heavy-ion therapy. It sheds light on the molecular mechanisms of radiation damage and may play an essential role in optimizing radiotherapy techniques in the future," said Prof. MA Xinwen from IMP, another corresponding author of this study.
This work was jointly supported by the National Key Research and Development Program of China, CAS, the National Natural Science Foundation of China, and the Ministry of Science & Higher Education of the Russian Federation.
DOI: https://doi.org/10.1103/PhysRevX.15.011053
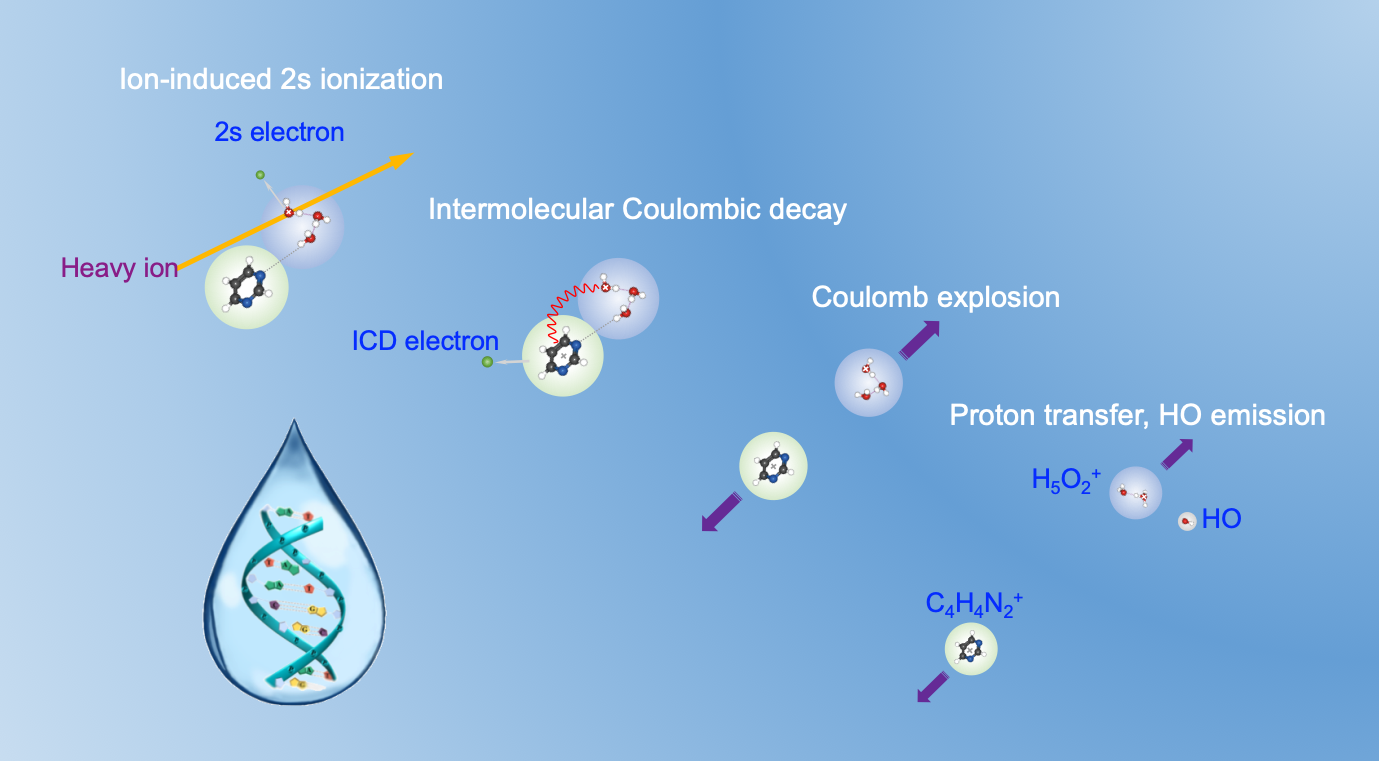
Figure 1. Relaxation mechanisms in the hydrated pyrimidine cluster after heavy-ion irradiation. (Image by IMP)

Figure 2. Reaction microscope inserted into the Cooler Storage Ring of the Heavy Ion Research Facility in Lanzhou. (Image by IMP)



 甘公网安备 62010202000713号
甘公网安备 62010202000713号


