Atomic spectroscopy of radioactive ions
Atomic and molecular laser spectroscopy and mass spectroscopy
The spectroscopic studies of atoms, molecules, and ions can provide important information for the physics, chemistry and relative sciences. The development of the new techniques, such as high-resolution laser, supersonic molecule beam, laser induced fluorescence (LIF) spectroscopy, emission spectroscopy and time-of-flight mass spectroscopy (TOF-MS) have made it possible to study those unstable molecules, including free radicals and molecular ions. Here, the transition metal compound radicals are produced by discharge-assisted laser ablation jet-cooled source (DALAS) [1]. The electronic structure of the radicals is investigated by optical spectroscopy [2, 3], and the chemical property of transition metal atom and stability property of the ionic cluster productions are investigated by combination of single-atom reaction and TOF-MS [4]. So far, the electronic structures of radicals S2, CuO, CuS, AuO, AuS, WO, WS, and MoO, as well as the reaction of carbonyl with single metal atom Mo, W, Re, Os, Ru, and Au, have been experimentally studied.

[1]. ZHANG Ji-cai, ZHAO Dong-mei, MA Xin-wen, YANG Jie,Spectroscopy and Spectral Analysis, 38, 3941 (2018);
[2]. J-C Zhang, W-L Zou, L. Zhang, D-M Zhao, X-W Ma, J. Yang, J. Quant. Spectrosc. Radiat. Transf. 256 (2020) 107314
[3]. Jicai Zhang, Dongmei Zhao, Qianlan Xiang, Xinwen Ma, and Jie Yang, 355, 96-100, (2019)
[4]. Yang Wang, Shiwei Cao, Jicai Zhang, Fangli Fan, Jie Yang, Hiromitsu Haba, Yukiko Komori, Takuya Yokokita, Kouji Morimoto, DaiyaKaji, Yves Wittwer, Robert Eichler, Andreas Türlere, and Zhi Qin, Phys. Chem. Chem. Phys., (2019) 21, 7147-7154
Atomic and molecular laser spectroscopy and mass spectroscopy
The spectroscopic studies of atoms, molecules, and ions can provide important information for the physics, chemistry and relative sciences. The development of the new techniques, such as high-resolution laser, supersonic molecule beam, laser induced fluorescence (LIF) spectroscopy, emission spectroscopy and time-of-flight mass spectroscopy (TOF-MS) have made it possible to study those unstable molecules, including free radicals and molecular ions. Here, the transition metal compound radicals are produced by discharge-assisted laser ablation jet-cooled source (DALAS) [1]. The electronic structure of the radicals is investigated by optical spectroscopy [2, 3], and the chemical property of transition metal atom and stability property of the ionic cluster productions are investigated by combination of single-atom reaction and TOF-MS [4]. So far, the electronic structures of radicals S2, CuO, CuS, AuO, AuS, WO, WS, and MoO, as well as the reaction of carbonyl with single metal atom Mo, W, Re, Os, Ru, and Au, have been experimentally studied.
[1]. ZHANG Ji-cai, ZHAO Dong-mei, MA Xin-wen, YANG Jie,Spectroscopy and Spectral Analysis, 38, 3941 (2018);
[2]. J-C Zhang, W-L Zou, L. Zhang, D-M Zhao, X-W Ma, J. Yang, J. Quant. Spectrosc. Radiat. Transf. 256 (2020) 107314
[3]. Jicai Zhang, Dongmei Zhao, Qianlan Xiang, Xinwen Ma, and Jie Yang, 355, 96-100, (2019)
[4]. Yang Wang, Shiwei Cao, Jicai Zhang, Fangli Fan, Jie Yang, Hiromitsu Haba, Yukiko Komori, Takuya Yokokita, Kouji Morimoto, DaiyaKaji, Yves Wittwer, Robert Eichler, Andreas Türlere, and Zhi Qin, Phys. Chem. Chem. Phys., (2019) 21, 7147-7154
Collinear laser spectroscopy on radioactive atoms
The last three decades have witnessed the great improvement in experimental determination of the isotopic and isomeric nuclear spin I, magnetic dipole moment mI, electric quadrupole moment Qs and changes in the mean-square nuclear charge radii d<r2> using the state-of-the-art laser spectroscopy methods[1]. The accuracy of laser spectroscopic data is so high that the nuclear parameters can be extracted without the need of a nuclear model[2-4].
In China, a new accelerator facility, High Intensity heavy ion Accelerator Facility (HIAF), is being constructed now in Huizhou. It will provide a magnificent variety of short-lived isotopes which are far away from the valley of nuclear stability. A low-energy ion beam of radioactive nuclides is designed in HIAF to perform the atomic and nuclear physics experimental studies. Collinear laser spectroscopy (CLS) and resonance ionization spectroscopy (RIS) are now being designed and developed to study the hyperfine structure splitting (HFS) and isotope shift (IS) of the neutral atoms and singly- and highly-charged ions. Collinear laser spectroscopy at HIAF is expected to be applied to study the rear isotopes close to the neutron or proton drip lines.
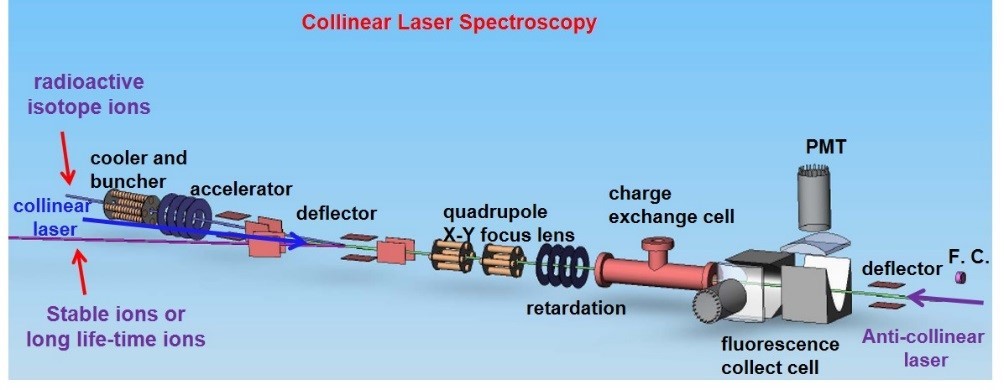
Diagrammatic sketch of the collinear laser spectroscopy experimental platform for the radioactive atoms at HIAF.
[1] H. –J. Kluge, W. Nortershauser, Spectrochimica Acta B 58 (2003) 1031-1045
[2] J. Sauvage et al., Hyperfine Interactions 129 (2000) 303-317
[3] W. Nortershauser et al., Physics Review Letter. 102 (2009) 062503
[4] H. Backe et al., Physics Review Letter. 80 (1998) 920-923
Laser spectroscopy of radioactive molecules
Radioactive molecules, in which one or more of the atoms are radioactive nucleus, provide a versatile physical environment to study the fundamental symmetries of nature and the interactions and properties of electron[1]. Especially, the radioactive molecules containing heavy and deformed nuclei offer high sensitivity for investigating parity- and time-reversal-violation effects[2]. With the accelerator HIRFL and HIAF facilities, we are planning to overcome the challenges to spectroscopically study the radioactive molecules, such as lifetimes of just a few milliseconds, production rates of less than 10-6 paritcal∙s-1, and laser cooling to sub-milli-Kalvin temperature. Also, the single-atom chemical reaction of heavy atom with oxygen or pentacarbonyl[3] will be performed on the same experimental platform.
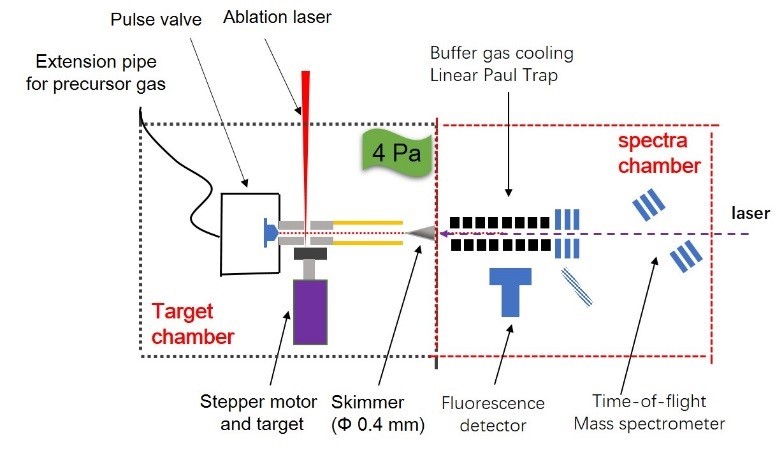
Diagrammatic sketch of the spectroscopic experiment platform for radioactive molecules.
[1]. Phys. Rev. Lett. 120, 142501 (2018); Nature 562, 355 (2018)
[2]. Nature 581, 396 (2020); Nat. Chem. 9, 578 (2017)
[3]. Phys. Chem. Chem. Phys. 21, 7147 (2019)
附件下载: